There are certain factors that increase the risk of developing polyps and subsequent colon cancer.
The risk of cancer increases as you get older, which is why colonoscopy screening starts in many countries at 50 years old (60 in New Zealand).
Information on bowel screening
The other thing you cannot change is your genes – (the risk passed down by your parents and their parents). There are certain genetic familial syndromes (such as Lynch syndrome, Familial adenomatous polyposis (FAP), sessile serrated polyposis syndrome) that confer a very high risk of developing cancer – and genetic testing should be performed.
If your mother, father, brother or sister has and/or had colon cancer, the risk of you developing colon cancer increases by over 2 times. If two of these people developed colon cancer, the risk goes up nearly 4 times (3.97 times to be exact). In addition, having a relative who had polyps is also believed to increase your risk.
A variety of modifiable and non-modifiable risk factors have been associated with polyp and colorectal cancer (CRC) development. In addition, some dietary factors may be protective.
Modifiable risk factors
It is estimated more than 50 per cent of incident CRC cases may be attributable to modifiable risk factors. This is shown by the more than 10-fold differences in incidence between developed and developing countries. Studies of immigrants show that just one generation after moving from a low-incidence to a high-incidence country, individuals assume the same risk of developing CRC as their adopted nation.
New Zealand-specific research has looked into six modifiable lifestyle categories. In order of population attributable fraction (PAF – the proportion of cases that could be prevented if that factor were eliminated), these are:
Obesity – PAF 9 per cent. In New Zealand, 30 per cent of adults have a BMI >30kg/m2. It is estimated that the risk of CRC is increased by more than 30 per cent in those who are obese compared with those with a normal BMI (<25kg/m2). Each 5kg/m2 increase in BMI is associated with a 5 per cent increase in risk of CRC.
Excess alcohol consumption – PAF 7 per cent. The risk of CRC increases by more than 44 per cent in heavy drinkers (more than five standard drinks per day). Of note, in New Zealand, 16 per cent of people have a hazardous drinking pattern.
More recently, studies have shown a J-shaped association, with light to moderate drinkers (up to two drinks per day) having a decreased risk of CRC, but heavy drinkers (more than two drinks per day or >30g of alcohol) having a significantly increased risk.
Red meat consumption – PAF 5 per cent. The mechanism by which red meat causes CRC is unknown. The current hypothesis is long periods of cooking releasing amines plus alteration of the gut microbiome. In New Zealand, 14 per cent of the population over age 15 eat red meat five or more times per week. There is a dose–response relationship with risk of CRC, with a 35 per cent increased risk when comparing the highest to the lowest red meat consumers.
Physical inactivity – PAF 4 per cent. The New Zealand Health Survey 2015/16 suggested 14 per cent of adults were inactive (defined as less than 30 minutes of physical activity in the last week). Physically active individuals have a 20–30 per cent lower risk of CRC compared with less active individuals, but the mechanism is not clear. It may be linked to more than just BMI reduction, as there is reduction in CRC risk even in the absence of significant weight loss.
Smoking – PAF 3 per cent. In New Zealand, 17 per cent of adults are smokers (smoked more than 100 cigarettes in a lifetime and currently smoking at least once a month). They have a 15 per cent increased risk of CRC compared with never-smokers.
Processed meat consumption – PAF 3 per cent. Nearly 9 per cent of the population eats processed meat five or more times per week. Similar to red meat, there is more than a 30 per cent increased risk of CRC when comparing the highest to the lowest processed meat consumers.
These findings are similar for Australia and the UK. Halving the prevalence of obesity, excess alcohol and red meat consumption is estimated to potentially reduce the number of CRC diagnoses in New Zealand by 318 per year.
Non-modifiable risk factors
Age – this is the most important determinant of risk. There is an exponential increase in risk of CRC with age.
Gender – men are more likely to develop CRC than woman. The incidence of colorectal neoplasia observed in men at a specific age is said to be found in women approximately 10 years later in life. Among women, risk of CRC increases steeply following menopause.
Family history of CRC – 20 per cent of people with CRC have two or more first-degree or second-degree relatives with CRC. Having a single first-degree relative increases the risk two to threefold, while two first-degree relatives increases the risk three to sixfold. Two second-degree relatives increases the risk twofold.
Inflammatory bowel disease – having a personal history of ulcerative or Crohn colitis increases the risk of CRC two to threefold. The risk of CRC increases with the duration and activity of the colitis. It conveys an estimated cumulative risk of developing CRC of 5–10 per cent after 20 years, and 20 per cent at 30 years.
Protective dietary factors
Fish – higher intake of polyunsaturated fatty acids, such as omega-3 fatty acids, has been associated with a lower risk of CRC. It is thought to counteract inflammation that leads to carcinogenesis.
Fibre – this may absorb faecal carcinogens, reduce colonic pH, and alter colonic transit time and bile acid metabolism. Ultimately, results from high-quality studies are inconclusive.
Calcium and vitamin D – these may have protective effects, including binding secondary bile acids and fatty acids, and inflammatory properties, respectively. Data on supplementation are inconsistent, but encouraging a full and varied diet with safe sunlight exposure can be recommended.
Summary
Modifiable risk factors are prevalent and may help to explain the number of CRC cases we are diagnosing. Collation of presenting symptoms, signs and risk factors should assist with decision-making regarding requests for further investigation. However, you should be aware that early CRC is asymptomatic, and even those with more advanced disease may not present in a classic manner.
______________________________________________________________________________
ADVANCED: Types of polyps:
Colorectal cancer genesis: Polyps of all shapes and sizes
The single layer of columnar colonic epithelium undergoes continuous cycles of turnover, completing a full renewal every five days. Based on this high rate of change, if any checkpoint of proliferative cell division malfunctions, abnormal cell growth may start, with a subsequent carcinogenic cascade of genetic mutation.
The majority of CRC develops from a focus of this growth in the mucosal lining of the bowel, termed a polyp. I describe polyps to patients as “warty outgrowths”. They come in all different shapes and sizes. Some are flat to the bowel wall, while others may be pedunculated with a stalk like a mushroom. They also vary in histological characteristics, from hyperplastic to sessile serrated to adenomatous to villous subtype.
Depending on a constellation of factors – most importantly, total number, size, location and histology – one can infer the potential risk of progression to CRC. This is defined as when malignant pass through the mucosal lining into the submucosa, where there is lymphovascular supply which may lead to metastatic spread.
Before this progression, when the depth of neoplastic invasion is small and therefore the risk of lymph node metastasis is zero, polyps can be completely removed through the colonoscope. Importantly, most polyps are not associated with clinical symptoms unless they are at a size where they are blocking the flow of stool or bleeding.
Adenomas
Adenomas are the predominant histological type, accounting for 60–70 per cent of all polyps. Approximately 30 per cent of the population will have at least one adenoma by the age of 50, and 3–8 per cent will have an advanced adenoma (defined as greater than 1cm in size), villous features, high-grade dysplasia (abnormal cancerous cells but confined to the mucosal layer, not entering the submucosa) or invasive cancer. Villous polyps account for only 5 per cent of adenomatous polyps but are eight to 10 times more likely to become malignant than tubular adenomatous polyps.
By definition, all adenomas have a focus of dysplasia, which is classified as “low grade” or “high grade” depending on the degree of abnormality of the cells and their nuclei. Adenomas progress through this order in a stepwise fashion, accumulating progressive dysplasia – this is termed the adenoma to carcinoma sequence (Figure 1).
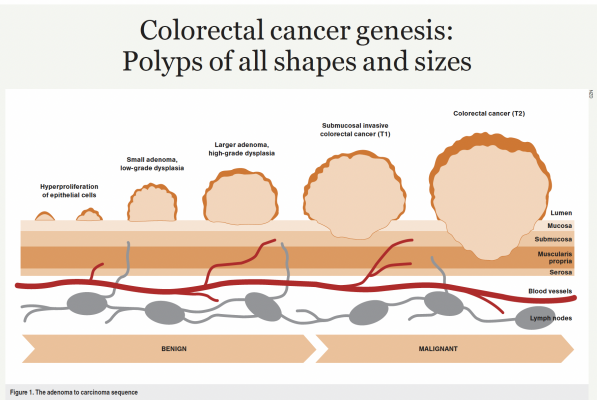
The natural history of untreated adenomas is understood from older studies performed before the advent of colonoscopy and its ability to endoscopically visualise and resect. Therefore, polyps were observed over time with periodic radiographic examination, and bowel resection performed once deemed to be worrisome. These early studies suggested the cumulative risk of diagnosis of cancer at the index polyp site at five, 10 and 20 years was 2.5, 8 and 24 per cent, respectively.
Clearly, more detailed and modern natural history studies are impossible to perform with ethical considerations, but it is generally accepted that there is a 5 per cent risk of an adenoma progressing to carcinoma, and this process may take five to 10 years.
Sessile serrated polyps/lesions
These are the other main histological class of polyps. Serrated polyps, named after the sawtooth appearance of the crypts when viewed microscopically, become cancerous through a distinctly different pathway. They account for 20–30 per cent of sporadic cancers. They are flat (sessile), translucent, frequently covered with mucus and are difficult to detect and recognise on colonoscopy. Because of this morphology, they are also not well seen with CT colonography (CTC) and frequently missed.
When detected, they may be incompletely removed due to their indistinct appearance and difficulty with assessment of the margin – 20 per cent of serrated polyps greater than 2cm are through to be incompletely resected. Because of this, they are estimated to account for 5–7 per cent of CRCs which occur after a complete colonoscopy and prior to surveillance – termed “interval cancer”.
Unlike adenomas, these polyps have the possibility of faster progression to cancer, particularly once they become dysplastic. Cases have been found to transform to cancer even within eight months.
Surveillance
Surveillance following a colonoscopy where polyps are removed is based on the observation that these individuals are more likely to develop recurrent polyps in the future. The landmark randomised trial of surveillance intervals defined an overall adenoma recurrence rate of between 32 and 42 per cent at three years after the initial polypectomy.
This risk for metachronous polyps is higher in individuals with a greater number of, and more advanced, polyps. This is reflected in the New Zealand Update on Polyp Surveillance Guidelines 2020, which dictates a repeat procedure in one, three, five or 10 years on this basis. For people over the age of 75, the potential benefits are less, and routine surveillance is often ceased. These intervals and suggestions are generally provided by the endoscopist, who may also consider the quality of the colonoscopy, including bowel preparation, and therefore the potential of missed lesions.
In New Zealand, surveillance every three to five years is suggested for patients with previously diagnosed CRC and for individuals with a significant family history of CRC. This is classified as one first-degree relative diagnosed at age 55 or under, or two first-degree relatives on the same side of the family diagnosed at any age.
More information on bowel cancer
Reference Aid: Doubeni et al. UpToDate, Patient education: Screening for colorectal cancer (Beyond the Basics) accessed 09/01/2021.
* Richardson A, Hayes J, Frampton C, Potter J. Modifiable lifestyle factors that could reduce the incidence of colorectal cancer in New Zealand. N Z Med J. 2016 Oct 16;129(1447):13–20.
Population attributable fraction (PAF) for New Zealand:
- 9% for obesity
- 7% for alcohol
- 4% for physical inactivity
- 3% for smoking
- 5% for consumption of red meat
- 3% for processed meat.
- PAFs differed by ethnic group and sex. In women, the highest PAFs were
- 19% for obesity in Pacific women
- 14% for obesity in Maori women
- 7% for physical inactivity in Asian women
- 8% for obesity in European/other women.
- In men, the highest PAFs were:
- 17% for obesity in Pacific men
- 14% for high alcohol consumption in Maori men
- 5% for physical inactivity in Asian men
- 9% for high alcohol consumption in European/other men.
